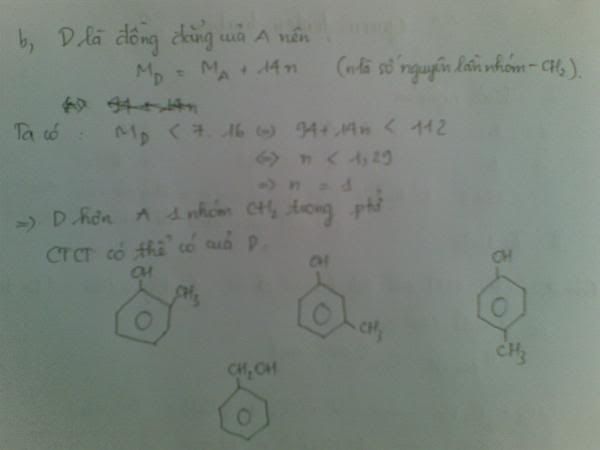
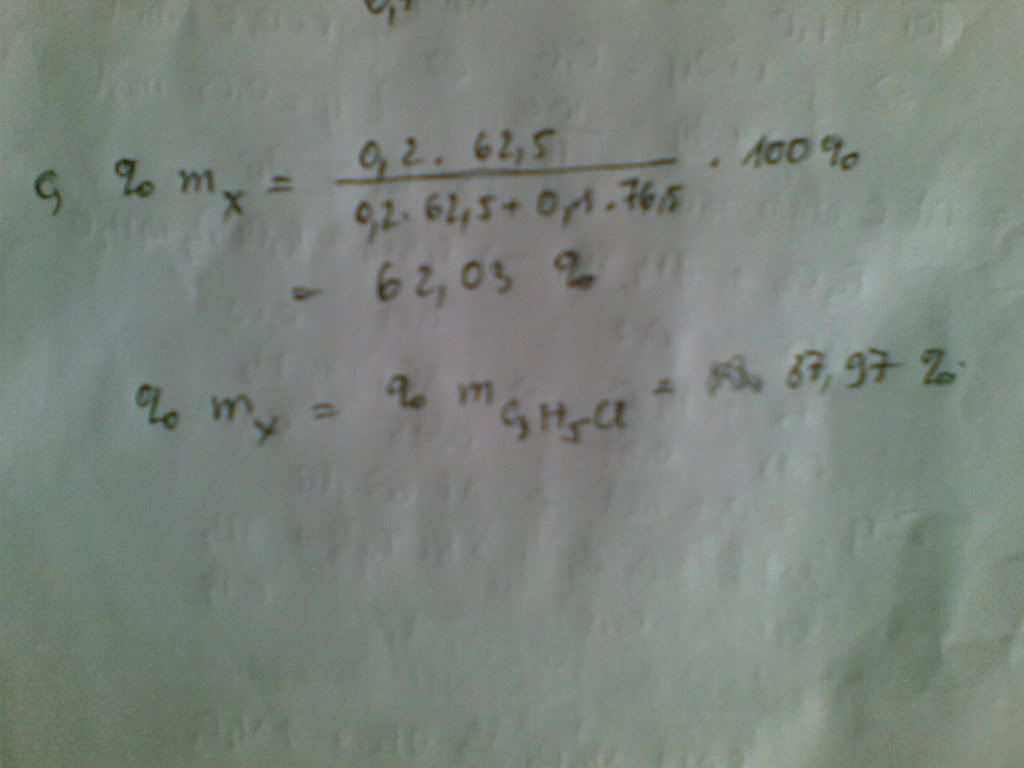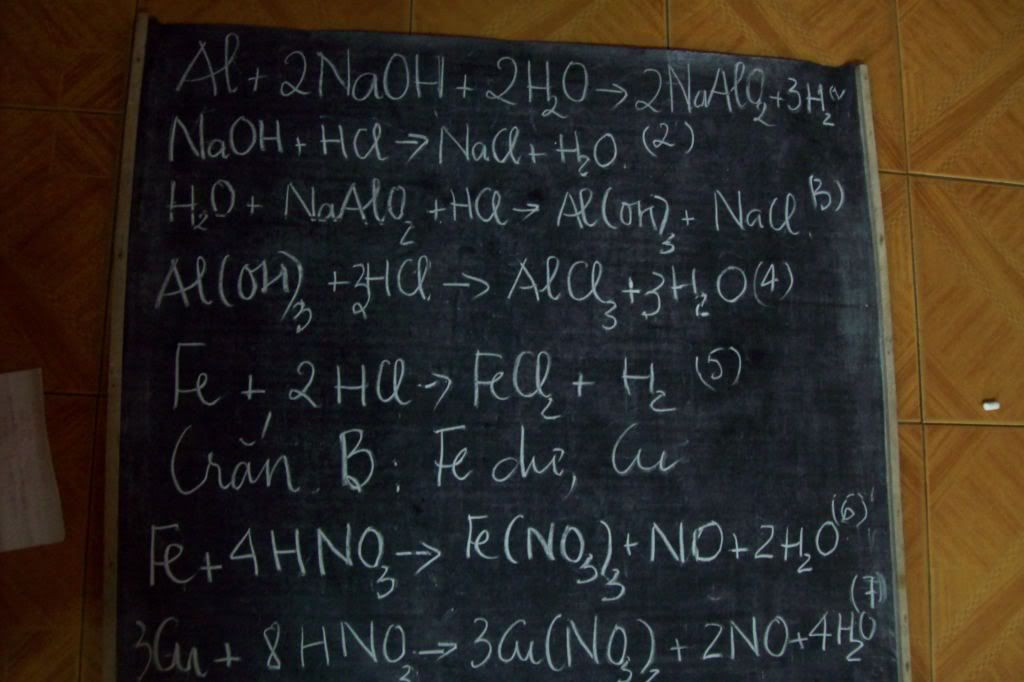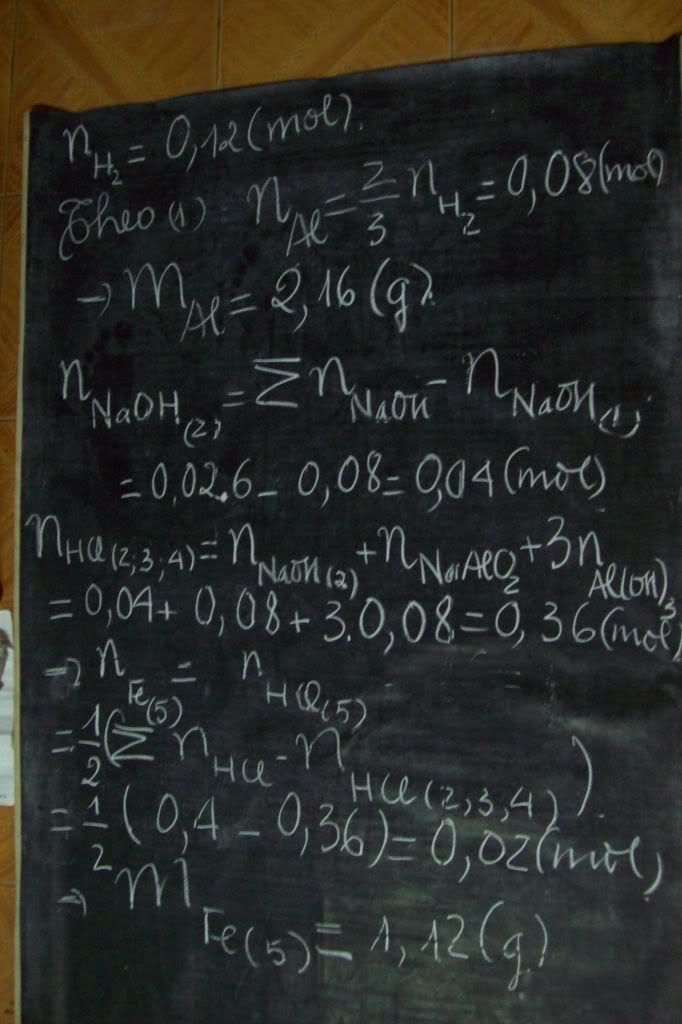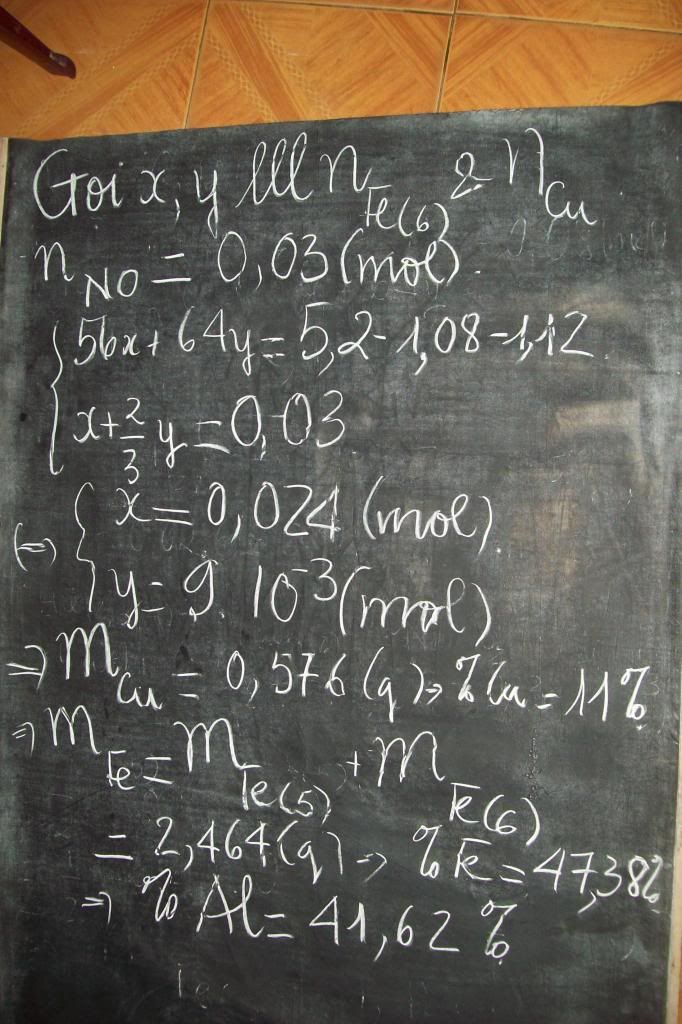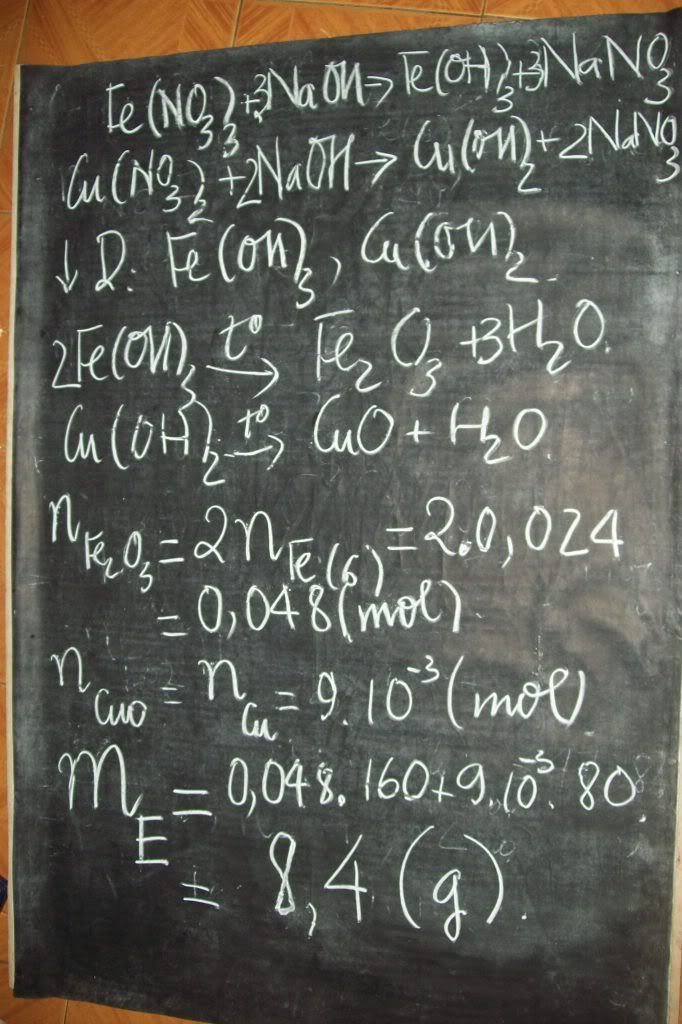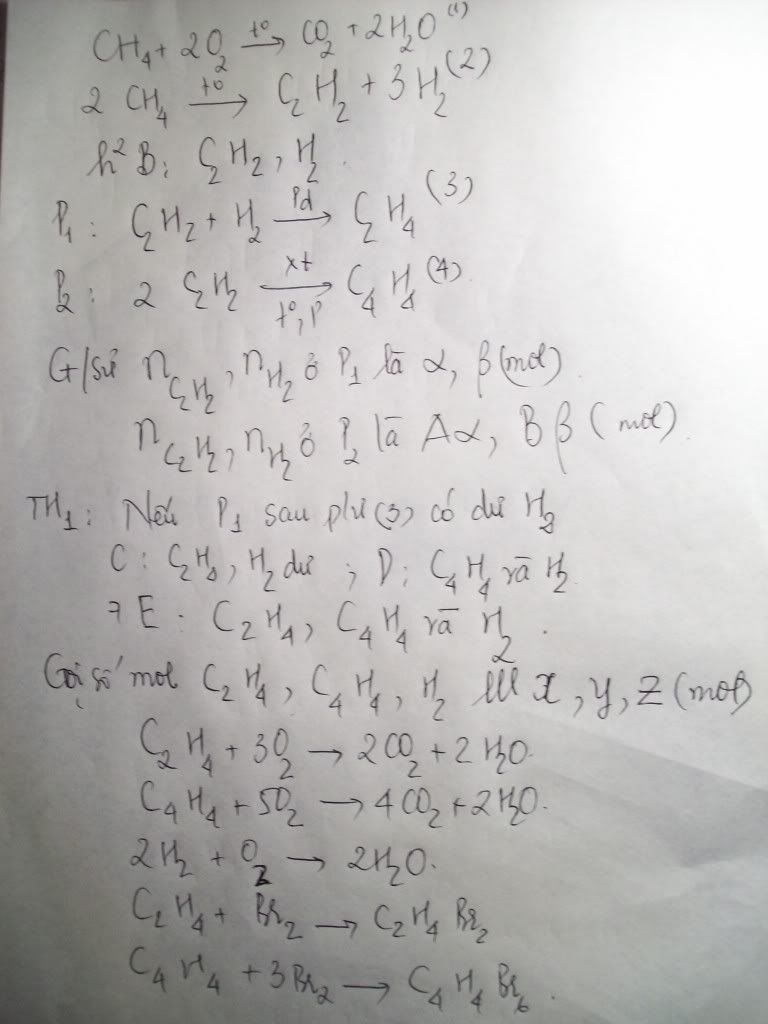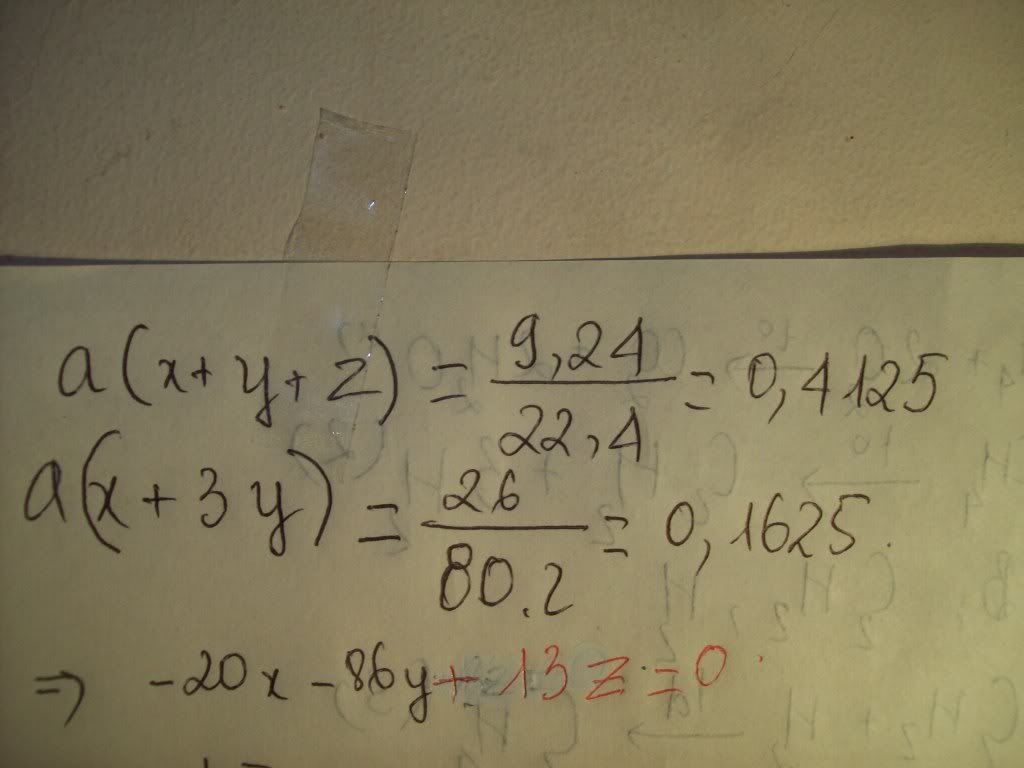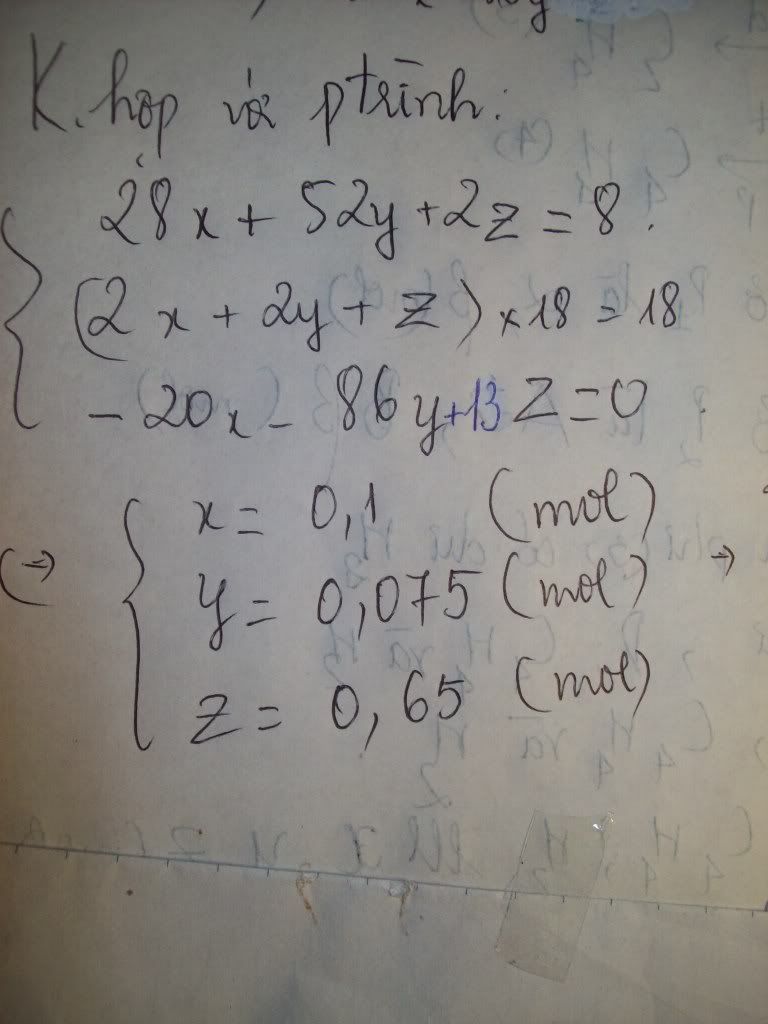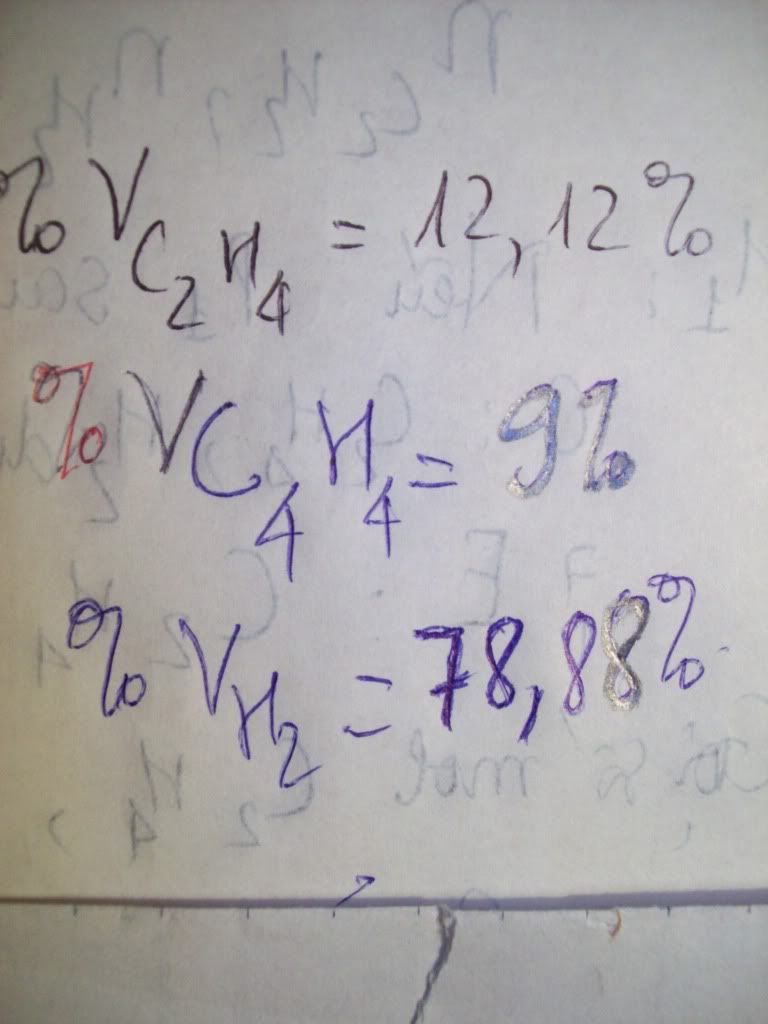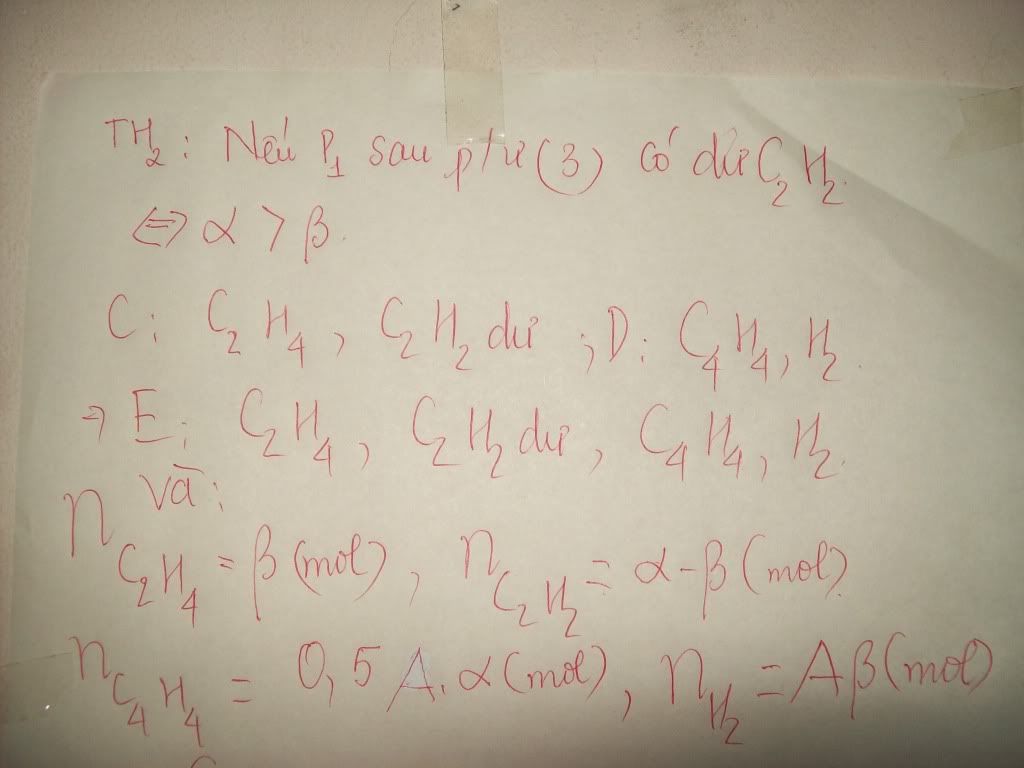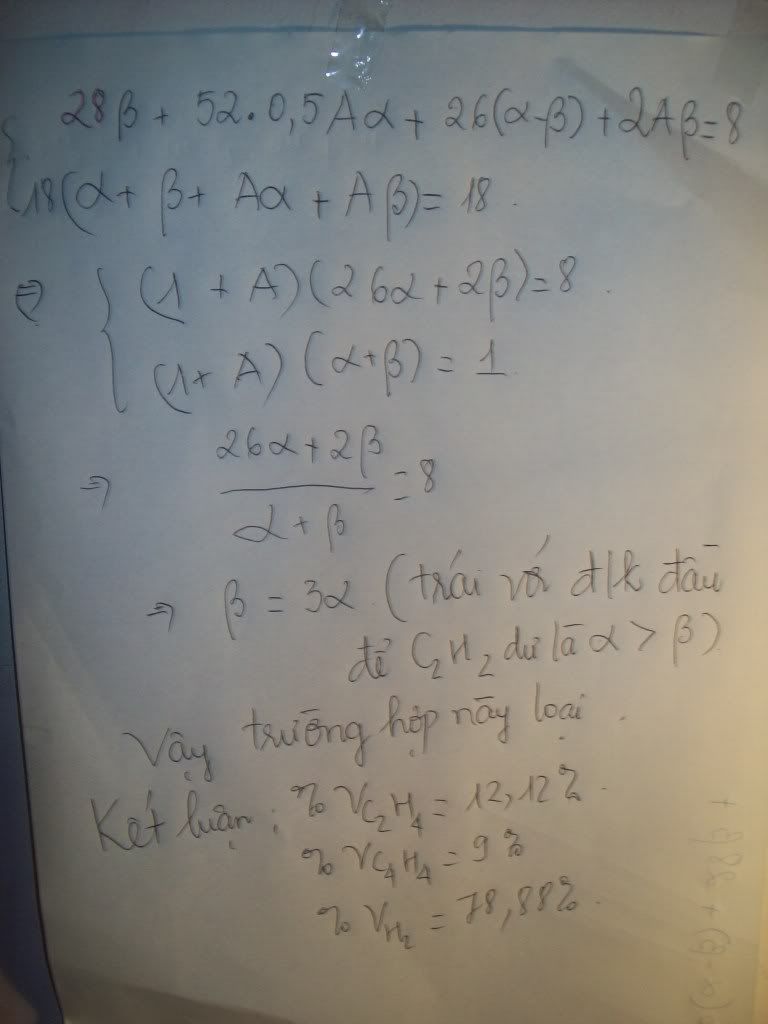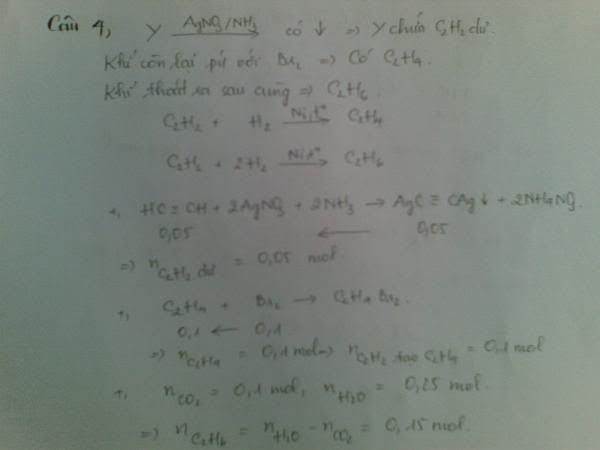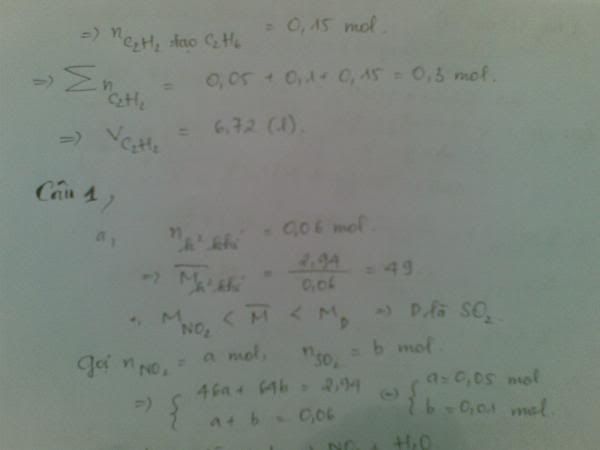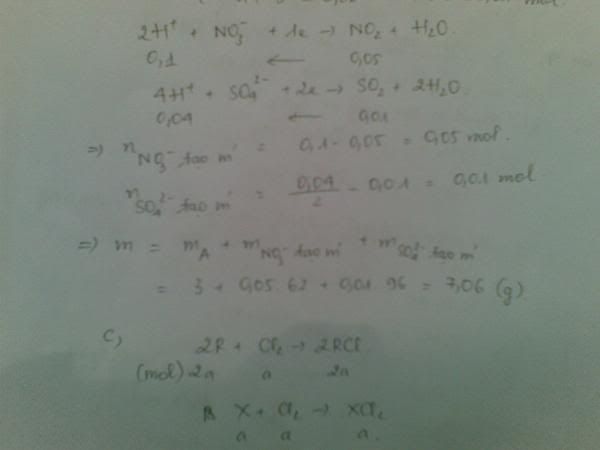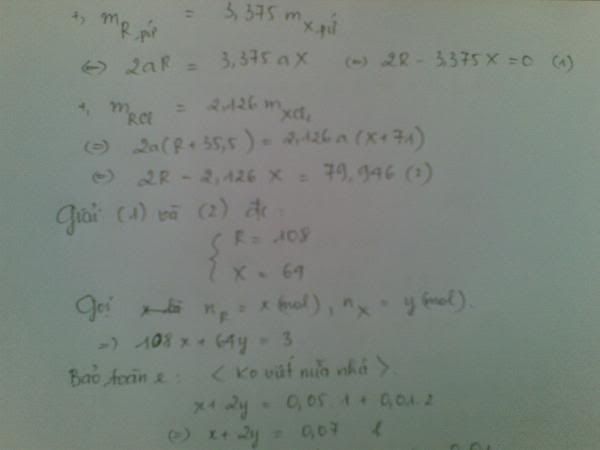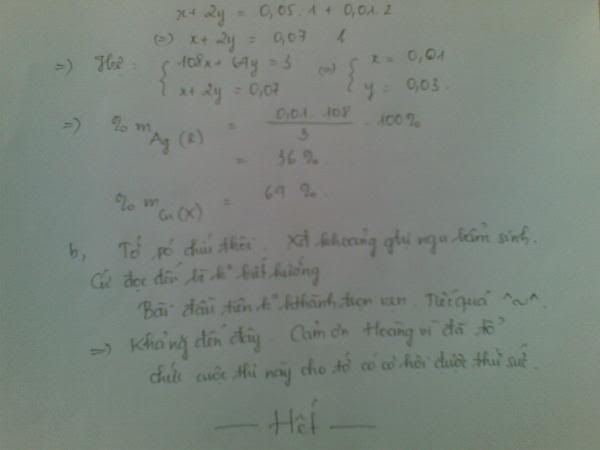1.Ta có:nH2=PV/RT=(1,5.9,846)/[0,082.(27+273)]=0,6 MOL
gọi nAl b đ=a mol,nFe3O4=b mol
các pt:
8Al+ 3Fe3O4-------->4Al2O3+9Fe
8x/3----x----------------4x/3----3x
nAl dư=a-8x/3 ,đặt a-8x/3=m
2Al+3H2SO4---------->Al2(SO4)3+3H2
m----3m/2--------------m/2---------3m/2
Fe+H2SO4----------->FeSO4+H2
y------y-----------------y---------y
ta có 3m/2+y=0,6
Al2O3+3H2SO4-------->Al2(SO4)3+3H2O
4c/3------4c--------------4c/3
Fe3O4+4H2SO4-------->FeSO4+Fe2(SO4)3+4H2O
3c-------------4.3c-------------3c----3c-
Al2(SO4)3+6NaOH------>2Al(OH)3+3Na2SO4
Al(OH)3+NaOH---->NaAlO2+2H20
Feso4+2NaoH----->Fe(OH)2+Na2SO4
y+3c-------------------y+3c----y+3c
Fe2(SO4)3+6NaOH---->2Fe(Oh)3+3Na2SO4
3c-------------------------------2/3c
2Fe(OH)3---->Fe2O3+H2O
2/3c---------------2/3c
Fe(OH)2--->FeO+H2O
y+3c----------y+3c
FeO+CO---->Fe+CO2
2/3c-----------------2/3c
Fe2O3+3CO--->2Fe+3CO2
y+3c----------------y+3c
2.gọi ct trung bình của X,Y là CzH2z-1Cl(trong đó ct X,Y : CnH2n-1Cl & CnH2m-1Cl)
n>=2 & n<z<m
pt
2 CzH2z-1Cl +(6z-1)/2 O2---->zCO2+(2z-1)/2 H2O+Cl2
theo bài ra ta có :a1=mCl2=10,65g-->n Cl2=0,15 mol
a2=mH2O,a3=mCO2.
TỪ pt trên -->nCzH2z-1Cl=2nCl2=0,15 mol,--->M(tb)=20,15/0,3=67,17 <->14z-1+35,5=67,17--->z=7/3=2,333
--->nCO2= 0,3. z=0,7 --->mCO2=0,7.44=30,8 g & nH2O=(2z-1)/2.nCzH2z-1=0,15.(2.7/3-1)=0,55mol---->mH2O=0,55.18=9,9 g
từ đk n<z<m,n>=2 nên -->n=2 do đó ct of X LÀ C2H3Cl
CTCT:CH2=CH-Cl
nC2H3Cl=x,nY=y mol
2 C2H3-1Cl +11/2 O2---->2CO2+3/2 H2O+Cl2
x mol----------------------------------------------x/2 mol
2 CmH2m-1Cl +(6m-1)/2 O2---->mCO2+(2m-1)/2 H2O+Cl2
y mol------------------------------------------------------------y/2mol
ta có (x+y)/2=0,15 hay x+y =0,3 (1)
nếu nC2H3Cl=x/2,ny=2y
2 C2H3-1Cl +11/2 O2---->2CO2+3/2 H2O+Cl2
x/2 mol----------------------------------------------x/4 mol
2 CmH2m-1Cl +(6m-1)/2 O2---->mCO2+(2m-1)/2 H2O+Cl2
2y mol------------------------------------------------------------ymol
ta có: x/4+y=0,15 <->x+4y=0,6 (2)
từ (1)&(2) giải hpt --->x=0,2 & y=0,1
mặt khác ta có z=(nx+my)/(x+y) 7/3=(0,2.2+0,1m)/0,3 ---->m=3
vậy Ctpt of Y là: C3H5Cl
ctct:CH3-CH=CH-Cl ,CH3-C=CH2,CH2-CH=CH2
/ /
Cl Cl
mX=0,2.62,5=12,5 g-------->%mX=12,5.100%)/20,15=62% --->%mY=100%-62%=38%